One of our NEJM Knowledge+ Internal Medicine Board Review questions has generated a fair amount of discussion and uncertainty. It asks the learner to select the best immediate response to a patient with a complication of central venous catheter (CVC) placement. The case-based question requires several cognitive steps, including considering the possible adverse events that could happen after putting in a CVC, interpreting physical examination findings, and identifying the appropriate interventions for ICU emergencies. Let’s begin by looking at the case.
The Case & Question
An 83-year-old man with a history of hypertension and chronic renal insufficiency presents with a chief complaint of altered mental status and a temperature of 39.5°C. His blood pressure is 104/64 mm Hg, his heart rate is 144 beats per minute, and his respiratory rate is 25 breaths per minute.
Initial laboratory testing reveals a leukocyte count of 26,500 per mm3 (reference range, 4500–11,000). Urinalysis is positive for nitrites and leukocyte esterase, shows 50 white cells per high-power field (0–2), and reveals gram-negative rods on Gram stain.
The patient is started on broad-spectrum antibiotics. Because of poor vascular access, a left-subclavian central venous catheter is placed (after several attempts); its position is verified by a portable chest radiograph.
Two hours later, despite continued resuscitation and medical therapy, the patient becomes acutely hypotensive with a blood pressure of 65/35 mm Hg. In addition, his supplemental oxygen requirement has increased from 2 to 15 liters per minute via a nonrebreather mask.
Physical examination reveals tracheal deviation to the right, absent breath sounds over the left chest, and dullness to percussion over the anterior and posterior left chest.
What is the most appropriate next step in managing this patient?
The Choices
- Needle decompression
- Placement of a large-bore thoracostomy tube
- Portable chest radiograph
- CT of the chest with intravenous contrast
- Immediate surgical thoracotomy and exploration
The Correct Answer
B. Placement of a large-bore thoracostomy tube
Complications of Central Venous Catheter Placement
The first step in working through this clinical scenario is to comprehensively review the complications that can be associated with central line placement in the subclavian position. For most clinicians, the greatest concern during CVC placement is the possibility of causing a pneumothorax. However, pneumothorax is not the only risk. CVC placement can also result in vascular injury, most commonly through arterial puncture, which can subsequently cause a hemothorax.
Hemothorax and pneumothorax are the two most likely complications in the case presented above. Both are slightly more common with subclavian than internal jugular CVC placement, and clinicians can reduce the risk for both by using ultrasound during the procedure. While the injury resulting in hemothorax and pneumothorax occurs during line placement, it usually takes some time for the patient to clinically deteriorate from these complications. Hemothorax can result in either tension physiology or hypovolemic shock, both of which will take several minutes to hours to manifest as blood has to accumulate in the pleural space. Pneumothorax can result in tension physiology as well — though the hemodynamic compromise from this, when a patient is on mechanical ventilation, is usually quicker than with hemothorax. This is because the positive pressure results in an increasing amount of air in the pleural space over several minutes.
Two other complications to consider when placing a CVC are arrhythmia and air embolism. Arrhythmias are generally caused by the guidewire entering the right ventricle and are usually aborted by pulling back the guidewire. Air embolism can occur once vascular access has been obtained with the introducer needle and steps are under way to place the catheter. Air can be entrained through the needle or through the catheter before capping all of the ports. Symptoms of air embolism range from dyspnea to cardiovascular collapse. Both air embolism and arrhythmias are complications that occur during the placement of the CVC, which was not the case with this patient, whose complications manifested 2 hours after the procedure.
Longer term risks of CVCs include infection, thrombosis, and venous stenosis — but, as mentioned above, this patient’s symptoms occurred 2 hours after the procedure, so none of these risks are reasonable concerns.
Having considered the possible complications and narrowed them down, the learner should now be focused on distinguishing between the two most likely complications in this case — pneumothorax and hemothorax — and then determining the best intervention.
Hemothorax vs. Pneumothorax
In an emergency in the ICU, the first step is to go to the bedside and examine the patient. In this case, there are some key physical findings that should help determine the cause of hypotension and distinguish between hemothorax and pneumothorax. The first notable finding is tracheal deviation to the right, away from the side where the procedure was performed. This should immediately raise concern for tension physiology as the cause of the acute drop in blood pressure. The deviation away from the side where the CVC was placed suggests that something is filling the pleural space and pushing the heart and mediastinum into the opposite hemithorax.
The lack of breath sounds does not help distinguish between hemothorax and pneumothorax as both would result in absent breath sounds. However, the percussion exam is helpful in discriminating between these two complications. In this case, there is dullness to percussion both anteriorly and posteriorly on the left. This result suggests that there is fluid in the pleural space, not air. A pneumothorax would be hyperresonant on percussion.
Overall, this patient’s physical exam findings in the setting of a recent CVC placement are consistent with a relatively rapidly accumulating hemothorax with resultant tension physiology.
Immediate Treatment
The most appropriate next step is to intervene on the hemothorax, to try to relieve the tension physiology caused by the rapidly accumulating blood. Blood needs to be drained with a large-bore chest tube (>36 French) because it is very likely to clot in a smaller caliber tube.
Needle decompression would be the correct intervention for a pneumothorax, but it would not effectively treat a hemothorax because the caliber of an angiocath would be much too small to effectively drain blood out of the chest — the flow would be so slow that there would be no impact on the tension physiology, even if the proceduralist tried to aspirate it as quickly as possible. Additionally, the needle is usually placed in the second intercostal space. Given that blood would be in the dependent thoracic zones, it would be unlikely to drain from something placed so high in the thoracic cage.
Although some patients do eventually need surgical exploration of the pleural space for either ongoing bleeding or incompletely evacuated blood, this would not be the best initial step because it would take too much time to get to the operating room.
Finally, what about a chest radiograph?
Imaging
In almost any institution, it takes several minutes (if not longer) to obtain a chest radiograph. In patients who are acutely decompensating with tension physiology from a hemothorax, treatment should not be delayed for a chest radiograph. A chest radiograph would help distinguish between pneumothorax and hemothorax, but the physical examination should be sufficient.
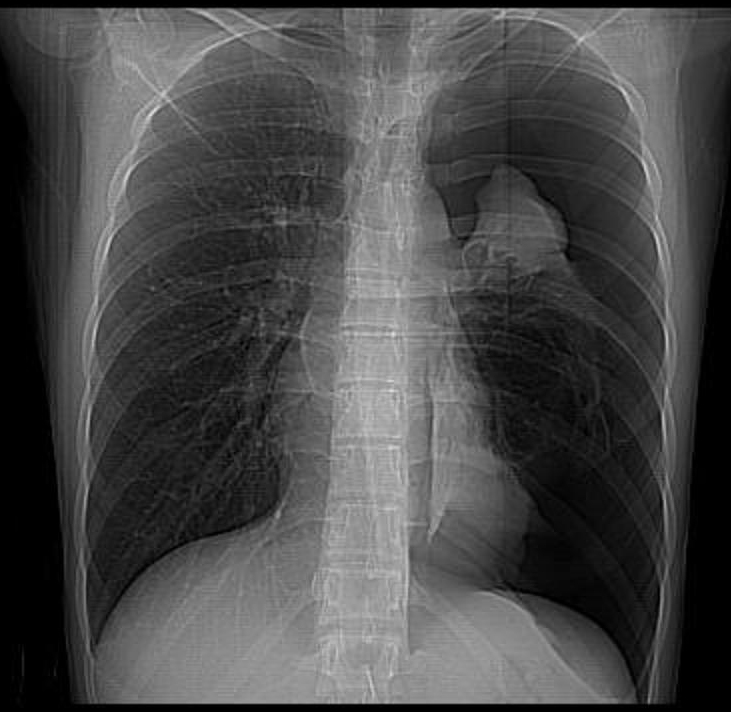
Example of a Hemothorax
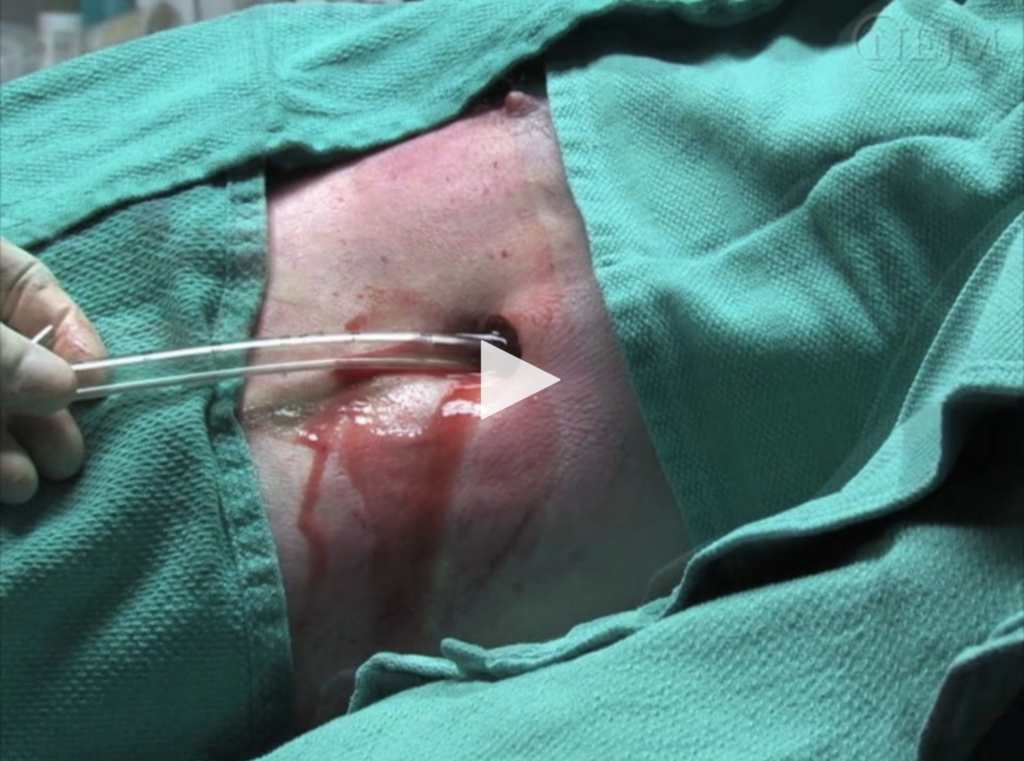
Example of a Pneumothorax
In both a hemothorax and a pneumothorax, we would expect to see the mediastinum and cardiac silhouette pushed to the opposite side of the thorax (i.e., away from the blood or air filling the pleural space). In the case of a pneumothorax, we would see a pleural line and a significant portion of the affected hemithorax without lung markings. In contrast, a hemothorax would opacify the entire affected hemithorax.
In many ICUs, a bedside ultrasound would be used to distinguish between these two etiologies. Ultrasound is often rapidly available on the unit, so it could be applied to the left chest. If there was a large anechoic collection between the chest wall and the lung, an effusion would be diagnosed. If instead, there was no lung sliding, pneumothorax would be highly likely. Lung sliding is the visible movement seen as the visceral pleura slides along the parietal pleura. This is seen in normal lung but would be absent in a pneumothorax where air now separates the visceral and parietal pleurae. The quick use of an ultrasound could confirm the physical exam findings and give the clinician greater confidence to move forward with the needed chest-tube placement.
Take Home
This case demonstrates the risk of tension hemothorax as a complication of CVC placement, the value of the physical exam in distinguishing between hemothorax and pneumothorax, and the importance of treating a hemothorax with a large-bore chest tube.
 Patricia A. Kritek, MD, is a practicing pulmonary and critical care physician and Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Washington, Seattle. She trained in Internal Medicine at Brigham and Women’s Hospital in Boston, Massachusetts, and completed her fellowship training in the Harvard PCCM program. She currently attends in both the Medical Intensive Care Unit and the Surgical Intensive Care Unit at UW Medical Center. Dr. Kritek is a clinician-educator who received her EdM from Harvard Graduate School of Education. She participates in teaching and advising of medical students, residents, and fellows. Her education work has focused on examining how best to provide feedback and guidance to medical trainees at all levels. Dr. Kritek has been writing for NEJM Journal Watch General Medicine since 2011 and is a section editor for NEJM Knowledge+.
Patricia A. Kritek, MD, is a practicing pulmonary and critical care physician and Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Washington, Seattle. She trained in Internal Medicine at Brigham and Women’s Hospital in Boston, Massachusetts, and completed her fellowship training in the Harvard PCCM program. She currently attends in both the Medical Intensive Care Unit and the Surgical Intensive Care Unit at UW Medical Center. Dr. Kritek is a clinician-educator who received her EdM from Harvard Graduate School of Education. She participates in teaching and advising of medical students, residents, and fellows. Her education work has focused on examining how best to provide feedback and guidance to medical trainees at all levels. Dr. Kritek has been writing for NEJM Journal Watch General Medicine since 2011 and is a section editor for NEJM Knowledge+.

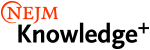
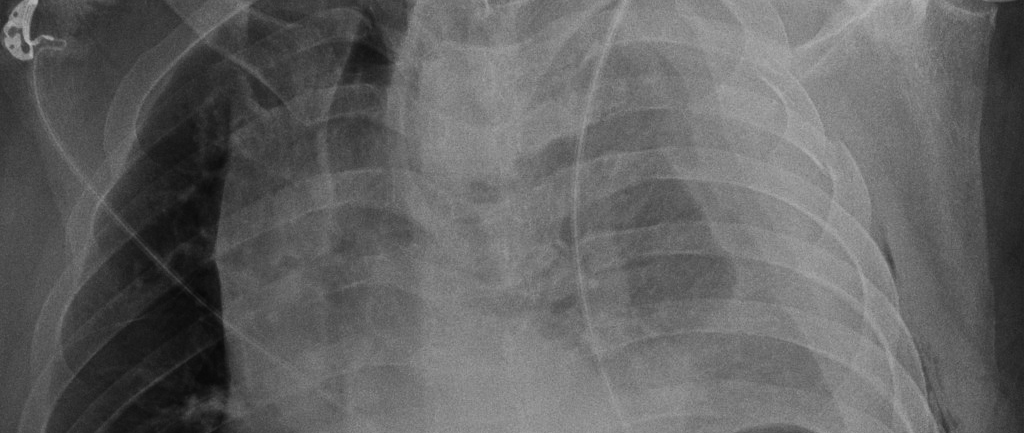

A combination of both in the form of hydro pneumothorax is also a common complication and may be difficult to distinguish clinically. It does respond well to insertion of an inter coastal chest drain as well
Immediate bilateral tube thoracostomies best. Is this the same patient? The earlier x-ray views appear to show at least one fractured rib on the left.
Several thoughts:
Ultrasound first, of course.
On second thought, U/S second, physical exam first.
In the setting of sole pneumothorax, the intravascular system is intact/at full volume. This causes a drop in return to the right heart. As the arterial side is pressurized and that pressure is unable to be drained in to the thorax (intrathoracic pressure > CVP) the venous system will distend. So look at the neck and forehead. If distended, air. Collapsed, hemothorax. This <1second observation, in conjunction with a focused physical will set the stage for verification by U/S. Finding venous distention, pop in a needle while you call for a chest tube tray. (Or maybe just needle, aspirate and observe.) For pneumothorax only.
and now the big question: when do you remove the line and who should be there?
This kind of depends on where the line is currently. It is possible that there was injury to an artery during placement (perhaps a first, errant pass with the introducer needle) but that the line was successfully placed in the vein. Or it could be that the injury occurred as the central line as placed into the subclavian artery.
There are several ways to try to make this distinction: send a blood gas off the line, transduce the pressure off the line or image to see where the line is (e.g., ultrasound or CT). I generally start with the first two.
If the line is in the artery, as your question I think implies, then my practice is to consult the vascular surgery team immediately and to work with them to get the line out as soon as possible.
“Both are slightly more common with subclavian than internal jugular CVC placement…”
I’d like to see a citation that references this fact. I’ve placed my share of IJ and SCV catheters (without US guidance) and this is not my experience. It is my (albeit anecdotal) experience the a SCV catheter can be placed more quickly than an IJ catheter, but it is more risky. I would suggest that a pneumo/hemo-thorax with IJ catheter insertion is far less common than the SCV approach.
Ultrasonography is the way to differentiate these pathologies
It is reasonable to drain small hemothorax (volume < 350 ml ) and stable patient. In this case just to use antibiotic and no drain ?.
Thanks, Julival Ribeiro