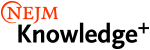This program has ended.
Information about the Covid-19 Vaccines can be found in the Vaccine FAQ on NEJM.org.
Covid-19 Vaccine Training: What Every Clinician Needs to Know is a free course in which you will explore the virology of SARS-CoV-2, the mechanism of action of Covid-19 vaccines, the efficacy and safety of currently available Covid-19 vaccines, and the practicalities involved in vaccine administration. This course reviews the principles of vaccine development and how the Covid-19 vaccines fit into this framework.
This free course will be available through June 30, 2021, unless there are major changes or advances in the development and administration of Covid-19 vaccines. Content will be reviewed and updated every 2 weeks.
A Learning Product That Adapts to Your Study Needs
NEJM Knowledge+ programs use adaptive learning technology to quickly assess the subjects you know well and identify the areas where you need reinforcement. The program then delivers more of what you need and less of what you already know. Adaptive learning enables you to spend your time efficiently, focusing on what you need to learn and review.
With this program, you will earn CME credits and ensure you understand the science and practicalities of the new Covid-19 vaccines.
- Earn up to 2.0 AMA PRA Category 1 Credits™
- Learn from content curated into five (5) topic areas:
- SARS-CoV-2 Virology
- Vaccine Development
- mRNA-based Covid-19 Vaccines
- Viral-Vector Covid-19 Vaccines
- General Considerations for All Covid-19 Vaccines
- Gain the knowledge you need to counsel your patients about Covid-19 vaccines
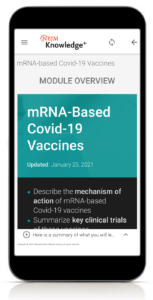 Mobile Access
Mobile Access
Covid-19 Vaccine Training is available wherever and whenever you have an internet connection and want to fit learning into your life. This program is accessible on all mobile and desktop systems and features one-step access from your mobile device — no need to download any apps.
A Free Online CME Course for Medical Professionals
Upon completing the Covid-19 Vaccine Training program, you will have the opportunity to print or download your CME Transcript for your records. Simply visit the Credit Center, within the platform, and follow the easy-to-navigate buttons to ensure you have a record of your completion.
Customer Support Notice
Editors
Education Editor, NEJM Group
Ole-Petter Riksfjord Hamnvik, MB BCh BAO, MMSc
Subject-Matter Experts
Varun K. Phadke, MD
Amy C. Sherman, MD
Reviewers
Jeffrey M. Drazen, MD
Rajesh T. Gandhi, MD
Daniel Randall, MD, MPH
C. Christopher Smith, MD
Activity Overview
Intended Audience
Any clinician who may be involved in advising patients on the vaccine, or prescribing/administering the vaccine.
Learning Objectives
- Describe the basic virology of SARS-CoV-2.
- Summarize adaptive and innate immune responses to SARS- CoV-2 infection.
- Describe antibody responses to SARS-CoV-2 infection and antibody testing methods.
- Summarize the various safety monitoring systems for vaccines before and after licensure.
- Describe the difference between vaccine efficacy and vaccine effectiveness.
- Describe the mechanism of action of mRNA-based Covid-19 vaccines.
- Summarize key clinical trials of mRNA-based Covid-19 vaccines.
- Describe how to administer mRNA-based Covid-19 vaccines.
- List the contraindications and safety profiles of mRNA-based Covid-19 vaccines.
- Describe the mechanism of action of viral-vector, whole-virus, and protein-subunit vaccines.
- List common adverse effects of non-mRNA-based Covid-19 vaccines.
- Recognize the limitations of current data on the efficacy and safety of on-mRNA-based Covid-19 vaccines.
Disclosure and Mitigation
The Massachusetts Medical Society (MMS) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) and must ensure independence, balance, and scientific rigor in their accredited continuing educational activities.
All individuals in control of the content for an MMS accredited continuing education activity must disclose all financial relationships with ineligible companies. An ineligible company is defined as companies whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
The following individuals disclosed relevant relationships with ineligible companies:
Rajesh Gandhi
– Advisory Board: Merck
Scott Calvert
– Shareholder: Puretech Health, Beyond Air, Contrafect, Translate
– Shareholder: Synairgen, Pulmatrix
All relevant financial relationships with ineligible companies have been mitigated prior to the beginning of this activity in accordance with ACCME and MMS policies.
Accreditation Term
Release Date: February 1, 2021
Termination Date: February 1, 2022
Content will be reviewed and updated every 2 weeks.
Accreditation Information
Accreditation Statement
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the Massachusetts Medical Society and Area9 Lyceum. The Massachusetts Medical Society is accredited by the ACCME to provide continuing medical education for physicians.
AMA Credit Designation Statement
The Massachusetts Medical Society designates this internet enduring material for a maximum of 2.25 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.