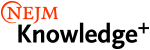Maximize the time you have for internal medicine board prep and lifelong learning with NEJM Knowledge+ Internal Medicine Board Review. Developed with time-strapped physicians like you in mind, our ABIM board review will help you successfully prepare for the Internal Medicine Board Exam or ABIM Longitudinal Knowledge Assessment (LKA), improve your medical practice, and earn CME credits and ABIM MOC points as you go.
Authentic Adaptive Learning Proven to Work
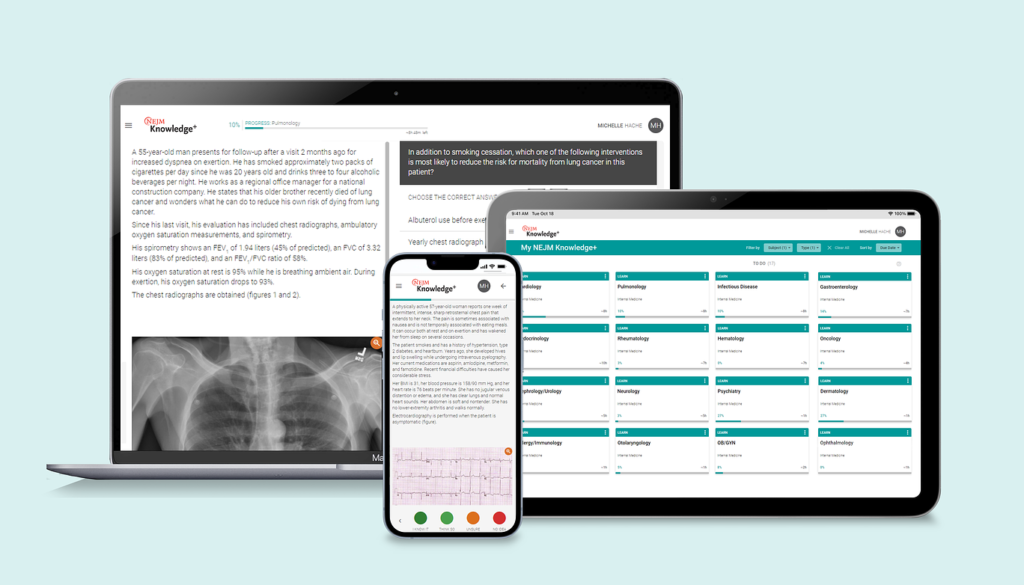
Other programs that claim to have adaptive learning, fall short. NEJM Knowledge+ Internal Medicine Board Review uses true adaptive learning technology to quickly identify areas of knowledge where you have gaps or need reinforcement. Unlike other board review programs, it delivers more of what you need to learn and less of what you already know, saving you valuable study time.
It does this by first assessing your existing knowledge and then leveraging AI to create the perfect learning path for you. This AI-driven technology was designed based on the best evidence about how people absorb and retain knowledge. The system also allows you to refresh your knowledge of completed learning modules or practice exams at any time to prevent memory decay. This allows you to maximize your time preparing for the Internal Medicine Board Exam or LKA, while also keeping your clinical knowledge sharp so you can deliver optimal patient care.
Most Comprehensive Internal Medicine Question Bank
NEJM Knowledge+ Internal Medicine Board Review delivers the most comprehensive question bank anywhere, along with two timed practice exams to help you gain the confidence you need to pass your board exam or LKA. Our question bank and practice exams are mapped to the ABIM blueprint and reflect the real-world challenges you face every day in your practice.
NEJM Knowledge+ Internal Medicine questions are:
- Just like questions you’ll see on the ABIM Exam and LKA, so you can practice answering questions effectively and within imposed time limits.
- Based on the best evidence available with access to further reading in guidelines, reviews, cases, and research.
- Created through a rigorous editorial process by experts at the top of their field and then reviewed by Internists who understand what is relevant and important today in Internal Medicine practice.
Best Internal Medicine Board Review Questions
The best internal medicine board review questions are designed to leverage the Testing Effect. Answering questions about new information primes your brain to receive and store it. And answering questions that require you to recall information you learned previously strengthens connections to it. By answering questions about new or previously learned information, you’ll more readily retrieve it from your stored memory in the future. In this manner, NEJM Knowledge+ Internal Medicine questions use the Testing Effect to strengthen your learning and make recall and retrieval easier.
How Many Questions are in NEJM Knowledge+ Internal Medicine Board Review?
NEJM Knowledge+ Internal Medicine Board Review has over 5,100 questions including multiple-choice, single-best-answer questions based on more than 3,200 unique learning objectives — the largest Internal Medicine question bank available. In the same format as the ABIM Board Exam and LKA, our board review questions include:
- The patient vignette or question scenario
- The lead-in or the question itself
- The answer options, which include one correct answer and several distractors
- Additional short and fill-in-the-blank questions based on the same learning objective as the case-based questions
Earn MOC Points and CME Credits While You Learn
You can earn AMA PRA Category 1 Credits™ with easy reporting by date that lets you keep track of what you’ve earned when.
With NEJM Knowledge+ Internal Medicine Board Review, you can also earn ABIM MOC points, and if you provide your ABIM number and DOB, we’ll automatically transmit your MOC points as you earn them. No paperwork. No hassle.
Get the Feedback You Need to Continuously Improve
NEJM Knowledge+ Internal Medicine Board Review tracks and reports your progress and performance, so you’ll know:
- How you’re performing and whether you’re on schedule to meet your goals
- Which areas are most challenging for you so you can focus on those topics
- How often you’re overconfident or underconfident to increase your metacognition making you a more effective learner and Internist
As your performance steadily improves and you reflect on your strengths and weaknesses, you can approach exam day or the LKA with true confidence.
Take Internal Medicine Practice Exams to Build Confidence for Exam Day
NEJM Knowledge+ Internal Medicine Board Review offers two (2) practice exams as another measure to track your performance and prepare you for test day. Questions in the practice exams align with ABIM exam questions and with the most common, relevant, and challenging clinical scenarios.
Each 2-hour online practice exam offers:
- 60 questions that mirror the format of the boards, so you’ll be ready for the real thing
- A pause feature that allows you to step away if you want a break
- Robust feedback on your performance: How you compare with others and how you performed in each subspecialty, along with key learning points, detailed feedback, and citations that include free access to NEJM articles, all available to you once you complete the exam.
Use the exams to direct where you want to start learning in the product, assess yourself partway through the learning, or get your timing down the week before your ABIM Board exam. It’s all up to you.
Online Internal Medicine Board Review: Learn on Your Schedule Whenever and Wherever You Like
Our online Internal Medicine Board Review doesn’t just adapt to your performance. It also adapts to your lifestyle. Because you can learn in bite-sized increments by answering as many or few questions as you like rather than wading through large chapters and reviews, you can fit learning in whenever you have a few minutes.
Use NEJM Knowledge+ Internal Medicine Board Review on your desktop or mobile device. Whenever you’ve got a free moment, NEJM Knowledge+ Internal Medicine Board Review is there with you — whether it’s in line at the supermarket, in the parking lot while waiting for your child’s soccer practice to let out, or during an unplanned minute between patients.
Get started with NEJM Knowledge+ Internal Medicine Board Review and accelerate your learning today!
Learn why Internal Medicine Question Banks are so effective.