On July 1, the NEJM Knowledge+ Question of the Week asked which testing was most appropriate for a sexually active 18-year-old woman who inconsistently uses condoms for contraception. The correct answer was to screen for chlamydia, gonorrhea, and HIV infection, but 50% of all respondents thought the patient should also be screened for infection with hepatitis B virus (HBV).
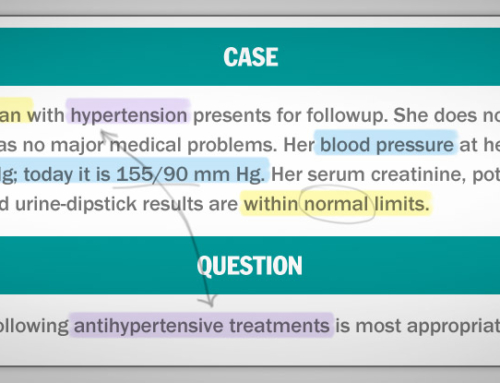
An 18-year-old woman wants to discuss long-acting, reversible contraception. She became sexually active 2 years ago. During the past 6 months, she has had two sexual partners who intermittently used condoms for contraception. She has not received the human papillomavirus vaccination. She is asymptomatic, in good health, and has no medical or surgical problems. Her physical examination is unremarkable.
Which of the following infections should this patient be tested for?
- Chlamydia and HIV (5%)
- Chlamydia, gonorrhea, hepatitis B virus, and HIV (50%)
- HIV and hepatitis B virus (12%)
- Chlamydia, gonorrhea, and hepatitis B virus (5%)
- Chlamydia, gonorrhea, and HIV (28%)
(n=10,155)
This pattern of responses — and the reader comments we received — prompted us to review the guidelines for hepatitis B virus (HBV) testing, which have recently been revised.
Recommendations on Screening for HBV in Asymptomatic, Nonpregnant Individuals
Although the U.S. Preventive Services Task Force (USPSTF) has long endorsed routine HBV screening for pregnant women, they have historically advised against it for asymptomatic, nonpregnant individuals, citing insufficient evidence. This summer, however, the USPSTF updated their recommendations in favor of screening certain populations for HBV infection:
- Individuals born in countries with a high prevalence of HBV infection (≥2%)
- HIV-infected individuals
- Injection drug users
- Household contacts and sexual partners of people with HBV infection
- Men who have sex with men
These populations were defined as high risk — and thus worth screening — on the basis of their current prevalence of HBV infection (≥2%) in the United States.
The USPSTF gave their new HBV screening recommendation a grade B, which indicates “high certainty that the net benefit is moderate or moderate certainty that the net benefit is moderate to substantial.”
The CDC guidelines on screening for HBV are similar to those of the USPSTF but also recommend HBV screening for individuals who are on hemodialysis or receiving immunosuppressive or cytotoxic medications.
The American Association for the Study of Liver Diseases (AASLD) guidelines for HBV screening include all of the groups highlighted by the USPSTF and CDC — but also include people who have multiple sex partners and those with a history of sexually transmitted infections.
Putting the HBV Screening Recommendations into Practice
So what do these recommendations mean for the case patient in our recent Question of the Week? She is a young woman with a history at least two sex partners and uses condoms only intermittently. This limited information allows us to establish that she does not meet the USPSTF or CDC criteria for HBV screening. However, she does meet a conservative interpretation of the AASLD criterion of “multiple sex partners” — and the USPSTF did leave the door open to screening a broader population than the groups they listed in their recommendations. They write:
Some persons with combinations of risk factors who are not members of one of these [high-risk] groups may also be at increased risk for HBV infection. Clinicians should exercise their judgment in deciding whether these persons are at sufficiently high risk to warrant screening. For example, screening is probably appropriate in settings that treat a large proportion of persons at increased risk, such as clinics for sexually transmitted infections; HIV testing and treatment centers; health care settings that provide services for injection drug users or men who have sex with men; correctional facilities; and institutions that serve populations from countries with a high prevalence of infection, including community health centers.
What’s a clinician to do? We asked Dr. Rajesh T. Gandhi, Associate Professor of Medicine at Harvard Medical School and Director of HIV Clinical Services and Education at Massachusetts General Hospital, who responded:
This patient is probably not at particularly high risk for HBV infection and would not meet the strictest criteria for HBV screening. However, if her sexual partners were in a high-risk group, that would sway me toward screening her for HBV. To that end, my next step would be to talk with her about the risk status of her partners and about her risk of acquiring HBV through unprotected sex. I would then proceed with the appropriate screening tests based on what I learned from the more detailed history.
Given the ambiguity in some of the HBV screening guidelines, we’ve revised this question in NEJM Knowledge+ to make it more straightforward. It now asks about an 18-year-old woman who has had only one sex partner.
As we all know, board-exam-style questions are rarely as nuanced as real-life cases, and physicians have to make judgment calls every day when interpreting guidelines for clinical practice. This particular Question of the Week gives us some insight into how physicians might be applying HBV screening guidelines in their practices. Our comment section below is open for your thoughts. We welcome examples and anecdotes from your own clinical experience.




In Italy this18th year old girl would have been vaccinated against the HBV virus, since vaccination became obbligatory since 1991. Regards
Given the very high prevalence of HBV carriers in certain populations worldwide (eg China) would it be worth adding the patient’s ethnicity (or country of birth) into the question?
Thank you for that suggestion, Dr. Huq. We’ll run it by our editorial team for consideration and update the question if necessary.